 |
 |
| |
Selected Development Project |
| |
 |
| Project Title |
Design and Synthesis of Bimetallic Complexes as Bifunctional Molecular Devices for Simultaneously Detection and Degradation of Industrial Pollutants |
|
| |
| Principal Investigator |
Dr CHOW Cheuk Fai Stephen |
|
|
|
|
|
| |
| Project Period |
|
|
| Objectives |
- To understand the mechanisms and factors affecting the efficiency of oxalic acid/oxalate detection and degradation by the cyano-bridged Ru(II)–C≡N–Zn(II) (RuZn2);
- To understand how the chemosensing and photo-Fenton catalytic properties of RuZn2 are controlled by the chemical properties of the metallic and organic functionalities;
- To explore other bimetallic systems, such as MA(II)–C≡N–MB(II) [MA = Fe(II), Ru(II), Os(II); MB = Fe(II), Fe(III), Mn(II), Co(II), Cu(II) and Zn(II)], for sensing and photodegradation of specific organic pollutants (such as cyanide and azo-dyes); and
- To study the applicability of such a technology to real industrial waste treatment of organic pollutants.
|
|
|
| Summary of Findings |
- A tetranuclear bimetallic complex, [RuII(tBubpy)(CN)4]2–[FeIII(H2O)3Cl]2×4H2O (1) has been synthesized and characterized. It was found to be a multifunctional device that can detect, signal amplify, and degrade an organic pollutant, oxalate.
- Chemosensing studies of 1 toward common anions: only oxalate selectively induces a naked-eye colorimetric and luminometric responses with method detection limits down to 78.7 and 5.5 ppm, respectively from 1.
- Photo-degradation studies of 1 toward oxalate: the dissolved organic carbon content of oxalate decreased and reached completely mineralization into CO2 within 6 hours.
- Signal amplification of 1 toward oxalate: through the photoassisted Fenton reaction by 1, methyl orange, an additional coloring agent, could be degraded so that the visual detection limit of 1 toward oxalate was magnified 50 times from 100 to 2 ppm.
- All the detection, degradation, mineralization and signal amplification were found applicable in real water bodies such as river, pond and sea water with excellent recoveries and relative standard deviation.
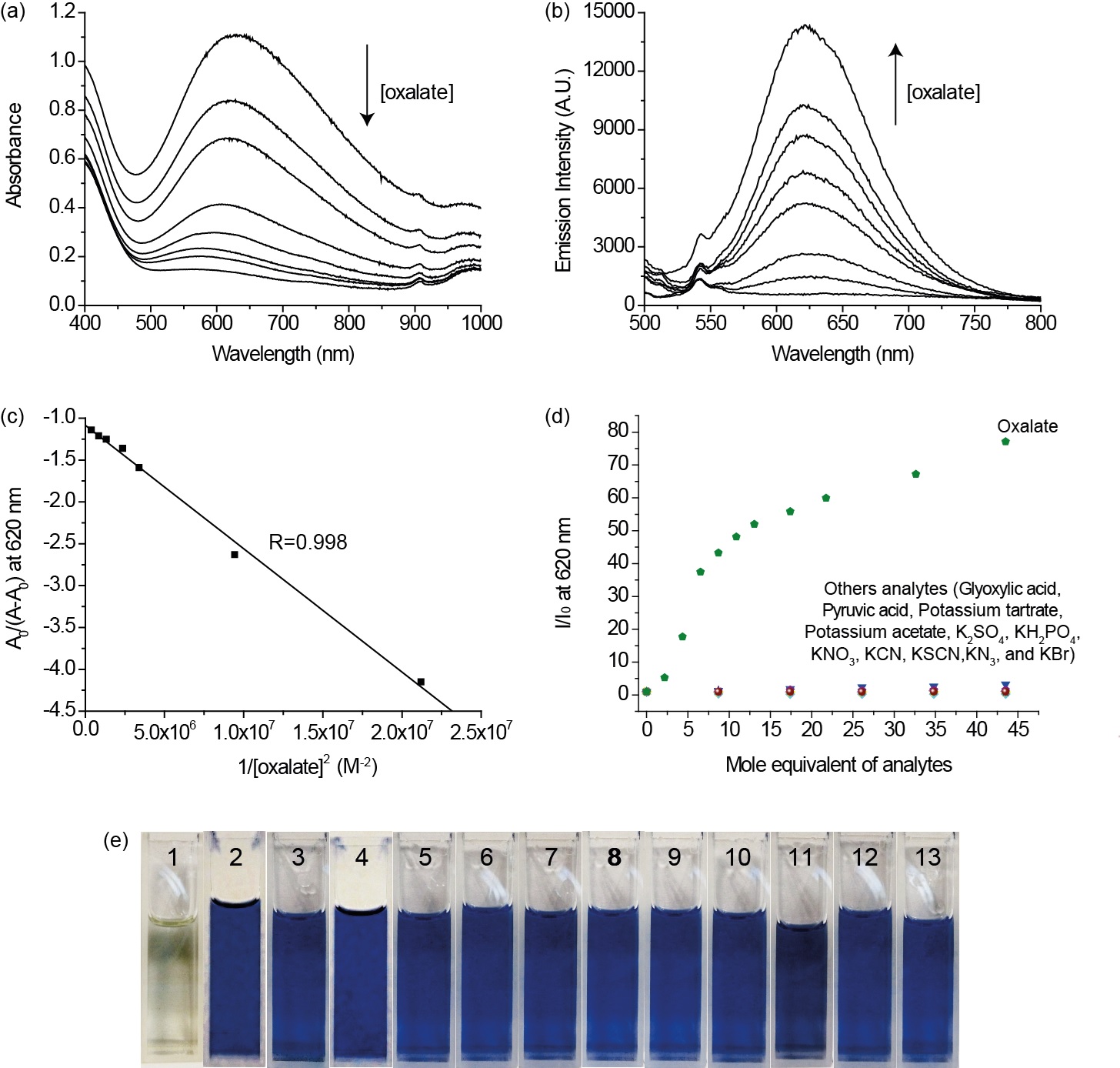
Figure 1. (a) UV–vis spectroscopic and (b) spectrofluorimetric titrations of 1 (1.08 ´ 10-4 M) with oxalate (0 to 3.24 ´ 10-2 M). (c) The best fitted A0/(A-A0) versus 1/[oxalate]2 plot with log K = 3.43 ± 0.03 at 620 nm (the slope and y-intercept are -1.084 and -1.47 ´ 10-7 M2, respectively). (d) Summary of spectrofluorometric titration (I/I0 at 620 nm) of 1 (2.17 ´ 10-4 M) to various analytes monitored as a function of the increase in their concentration. (e) Photos of the colorimetric responses of complex 1 (2.17 ´ 10-4 M): (1) 1 + oxalate, (2) 1 only; (3-13) 1 + glyoxylic acid, pyruvic acid, potassium tartrate, potassium acetate, K2SO4, KH2PO4, KNO3, KCN, KSCN, KN3, and KBr. All luminometric and colorimetric responses were recorded in aqueous ethanol (1:2 v/v) (1.00 mL of aqueous KCl/HCl buffer at pH 1.5 + 2.00 mL of ethanol) at 298 K. Excitation at 468 nm.
Table 1. Results of oxalate detecting/degrading/signal amplifying lake, river, and underground water samples with 1.
Detection |
Water samples |
Oxalate added
(μg/L) |
Oxalate found
(ppm) |
Recovery
(%) |
RSD (%) |
lake |
33.3 |
31.5 ± 0.9 |
94.6 |
2.76 |
river |
33.3 |
38.6 ± 1.0 |
115.8 |
2.60 |
underground |
33.3 |
30.8 ± 0.7 |
92.4 |
2.24 |
Degradation |
Water samples |
Oxalate added
(μg/L) |
Oxalate left
(ppm) |
DOC95
(min) |
lake |
767.0 |
0.0 |
175 |
river |
767.0 |
0.0 |
205 |
underground |
767.0 |
0.0 |
200 |
Amplification |
Water samples |
Oxalate added
(μg/L) |
Oxalate found
(ppm) |
Recovery
(%) |
RSD (%) |
lake |
10.0 |
10.1 ± 0.07 |
101.4 |
6.42 |
river |
3.3 |
3.5 ± 0.09 |
103.9 |
9.18 |
underground |
13.3 |
12.7 ± 0.07 |
95.1 |
6.89 |
|
Impact |
- The widespread use of organic chemicals in industries has led to significant wastewater pollution and serious health problems throughout the world.
- Oxalic acid, cyanide, azo-dyes, amines, and organophosphate pesticides are other common industrial wastes.
- Excessive accumulation of oxalic acid in the human body can cause a variety of health disorders, such as renal failure, urinary stone disease, and pancreatic insufficiency.
- Cyanide depresses the central nervous system by interacting strongly with the heme in cytochrome-a3.
- Organophosphate pesticides inhibit inhibit the activity of the enzyme leading to human health hazards such as contraction of pupils, profuse salivation, convulsions, involuntary urination, and defecation by asphyxiation.
- In this context, a smart molecular device that can detect, signal amplify, and degrade the industrial pollutants is highly desirable.
|
Output |
- Chow, C. F., Ho, P. Y., Gong, C. B. (2014). Ru(II)-Fe(III) Bimetallic Complex as a Multifunctional Device for Detecting, Signal Amplifying, and Degrading Oxalate. Submitted.
|
| Biography of Principal Investigator |
Dr. Stephen Cheuk-Fai Chow received his B.Sc. (Applied Chemistry, 1st class honour) and Ph.D. from the City University of Hong Kong in 2000 and 2005 respectively. After graduation, he obtained a Croucher Foundation Fellowship and did his post-doctoral fellowship with Nobel laureate Jean Marie Lehn at the University of Strasbourg, France (2005-2007). His studies continued with Prof. Chi-Ming Che (The Chinese Academy of Sciences) and Prof. Michael Hon-Wah Lam at The University of Hong Kong (2007-2009) and The City University of Hong Kong (2009-2010) respectively. Dr. Chow joined the Department of Science and Environmental Studies of The Hong Kong Institute of Education as Assistant Professor in 2010 and became Associate Professor in 2013. Dr. Chow received The President’s Award for Outstanding Performance in Research (Early Career Research Excellence Award) from The Hong Kong Institute of Education in 2013. |
Funding Source |
Early Career Scheme |
|
|
|
 |
|
 |


